Defining Quality and Testing Specifications for Cannabis Cultivators
There are many definitions of quality when it comes to cannabis. In fact, determining a universal standard for judging the overall quality of a cannabis product is one of the things over which the industry has struggled to agree. The one thing we can agree on is that it’s definitely more than just the THC potency.
The Importance of Product Testing for Micro Operators
In order to set your facility up for success as a micro operator, you need to prioritize cannabis product testing.
Lab testing is how we verify product safety, whether you’re producing whole flower, isolates and distillates, or edibles. But it’s important to remember that cannabis testing is about more than just meeting regulations; it’s about setting a standard that will benefit both consumers and the bottom line.
Navigating Lab Testing and Product Development
Proper lab testing is a critical facet of product development, on-going product manufacturing, and supply chain management. Obtaining accurate analytical data equips you to make informed decisions about every step along the manufacturing process.
Fortunately, you don’t have to do it alone. Laboratories like Labstat offer guidance and intelligence from the industry’s leading licensing, regulatory, compliance, and testing experts on how to set your business up for success.
Defining “Quality” in Cannabis Products
The trouble with a word like “quality” is that everyone has their own definition of what it means.
Some experts’ definitions and standards for quality are related to objective facts, while others are more related to subjective feelings and experiences. As consumer product makers, it can’t be denied that the quality of your cannabis product is also determined by the perception that a customer develops based on their personal experiences.
The dictionary defines quality as; the standard of something as measured against other things of a similar kind; the degree of excellence of something.
Understanding Quality Standards and Specifications
Quality standards are designed to ensure companies meet the minimum requirements to become an integral part of almost every industry, from food to automotive to healthcare and now, cannabis.
However, setting quality standards and specifications for your products isn’t just about avoiding product recalls or gaining market share over your competitors. It’s also about ensuring safety and consistency, delivering on a promise, and meeting the customer’s expectations every time they use your product.
Inevitably, the brands that invest in developing their product standards and testing specifications are better equipped to produce high-quality, innovative products that stand out in the crowded marketplace.
What is a Specification?
A specification is a technical document that defines a set of criteria a product needs to meet to be considered acceptable for its intended use.
Generally specifications are needed throughout the raw materials and manufacturing stages of each product. They will also be used to define the minimum quality standards that your final product must meet to be released to market.
Setting Quality and Testing Specifications
When it comes to defining quality and testing specifications, it is important to set your business up for success from the beginning. This means figuring out the quality parameters and testing specifications for your products before they end up on the shelf.
Fortunately, the right cannabis testing lab will be able to help you navigate the elements of determining your specifications, as well as understanding the impacts.
Your lab can also help in creating an organized quality system, with a set of well-defined specifications for your products. Once you have defined the quality parameters for your product, you must then determine which test should be included in your specification to verify they meet the requirements. Work with your lab is ensure which tests will provide the insights and data you need.
What Should I Include in My Specifications?
Your specifications must include the following:
- Required testing
- Reference to the analytical test method
- Acceptance criteria
- Product name
- Part number
- Revision number of the specification
- Effective date
- Approvals
These critical quality attributes are generally expected in every specification:
- Identification
- Assay
- Impurities
Additional attributes that can be included at discretion:
- Terpenes
- Authenticity
The specification document should define:
- Parameters controlling overall product quality according to internal standards and Health Canada requirements
Additional Health Canada Requirements
Health Canada may also require certain critical quality attributes to be included on your specification.
For example, a dried flower specification must include…
- Identification
- Breakdown of cannabinoids
- Impurities
- Microbiological examination
In addition to compliance testing, you’ll also want to include quality control specifications for any known attributes or characteristics that can affect the final product quality.
For example, testing for terpene content won’t impact the HC requirements, but it does affect the sensory experience the customer has while consuming the product.
Include Product Attributes in Your Specifications
The attributes you identify can also apply to brand guarantees regarding potency. For instance, a producer might require their whole product sku to have 22% THC or higher as a minimum standard. Or, they may want to ensure all the cultivars labelled “high CBD” have a verified 10% CBD or more.
These are internal quality specifications that should be included in your specifications documentation. You can organize this document in several ways; just be sure to keep it simple. It’s helpful to keep your specifications tabular so they are easy to read and understand.
Methods and Validation in Cannabis Testing
Once you’ve determined your quality specifications, you’ll work with your lab to identify which test methods will help you validate the quality of each batch you produce.
At this stage, the importance of method validation in cannabis testing cannot be underestimated. Health Canada requires that the test methods used to assess your product’s quality be validated. Specifically, Health Canada requires methods for cannabinoid assay, pesticide residues, residual solvents, and microbiological examination to be validated.
What are the Attributes of a Good Test Method?
A well-written and organized test method is a leading indicator that the results your lab is providing will be accurate and reproducible. A good test method will be well organized and will use an economy of words to ensure instructions are clear, concise, and correct.
The Role of a Monograph in Cannabis Quality Assurance
Methods can be a combination of internal test methods, external methods from your testing laboratory, or pharmacopeial methods. A monograph can be a helpful tool in quality assurance; it combines specifications with all testing methods used.
In fact, if there were a standard framework and set of monographs for testing cannabis products, producers and consumers would have a level of assurance in product safety regardless of what lab does the testing.
How to Define Your Acceptance Criteria
You’ve determined your quality specifications and attributes, you’ve identified the necessary tests, and now you must define the acceptance criteria. This outlines the minimum standards your product must meet to be deemed acceptable for release.
Acceptance criteria isn’t simply about pass or fail; it sets the significance of the reported number in your Certificate of Analysis. Certain thresholds will be determined by you and your quality assurance team, others are set by a number of governing bodies like Pharmacopeia.
Acceptance criteria can be…
- Numeric
- Descriptive
- A requirement to record or report the result
- It can represent either a single value, a range, or a comparison (e.g., visual examination)

It is critically important that the acceptance criteria be a balance of quality and manufacturability. Too tight of a specification range and good batches will be unnecessarily rejected. If your criteria is too wide, bad batches will be accepted and reflect poorly on your brand.
A good rule of thumb is that the less precise the measurement, the greater the variability in released product.
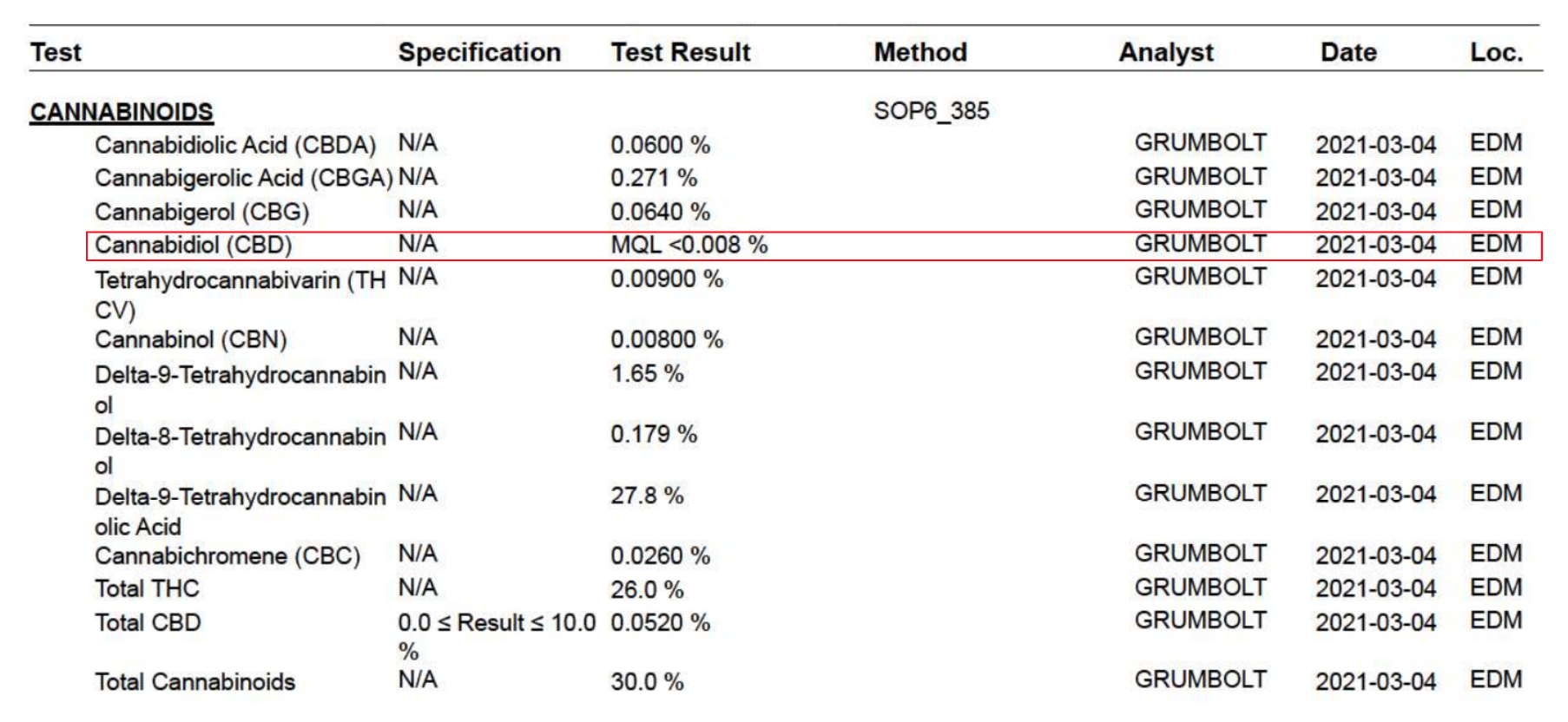
How to Analyze the Certificate of Analysis
Much like the specification, a certificate of analysis should provide…
- List of all tests performed
- Acceptance criteria
- Result obtained
- Test method
- Who performed the test
- Date the test was conducted
- Location of the test
This is how you can verify that every test prescribed within your specification document appears on the certificate of analysis.
The certificate of analysis must also allow the reader to determine if each of the defined attributes have met the acceptance criteria. This is accomplished by including both the result and acceptance criteria limits.
In the example below, we can see that Total CBD content was specified to be NLT 0.0% and NMT 10.0%. From this certificate of analysis we can determine the product met specification with a result of 0.0520%.
Understanding Method Validation and Data Reporting
Not all methods of validation are created equal. It is important to understand how data is being reported on your certificates of analysis.
For example, whole flower is currently graded based on the cannabinoid content, which is expressed as a percentage. Do you know what that percentage actually represents? Cannabinoid potency testing can be done in different ways, which is how some are gaming the system to inflate THC values.
This is why it is essential to know what your lab is doing and how they are producing their results.
Competing on Quality in the Cannabis Industry
With mounting competition and dwindling margins, your ability to compete based on quality has never been more important. Ensuring that you’ve properly defined your quality specifications is the first step to building a world-class portfolio of products.
If you’re just starting out, build these aspects into your business operations early. If you’ve already begun manufacturing, partner with a cannabis testing laboratory that can help you implement the quality processes and documentation that will ensure your long-term success.
As always, reach out to our cannabis testing experts if you need testing or help with your specification.



