The 78th Tobacco Science Research Conference (TSRC) devoted a full session to risk assessment, and it delivered practical tools, candid debate on 2024 FDA CTP tox memos (“Genotoxicity Hazard Identification and Carcinogenicity Tiering of Constituents in ENDS Premarket Tobacco Product Applications”, “Calculating Excess Lifetime Cancer Risk in ENDS Premarket Tobacco Product Applications”), and a clear call for more transparency from regulators and industry.
This year’s risk assessment sessions highlighted recurring themes: the need to avoid inflated Excess Lifetime Cancer Risk (ELCR) estimates, the importance of applying weight-of-evidence (WoE) approaches, and the integration of quantitative risk assessment (QRA) with in vitro toxicology to generate more decision-relevant outcomes.
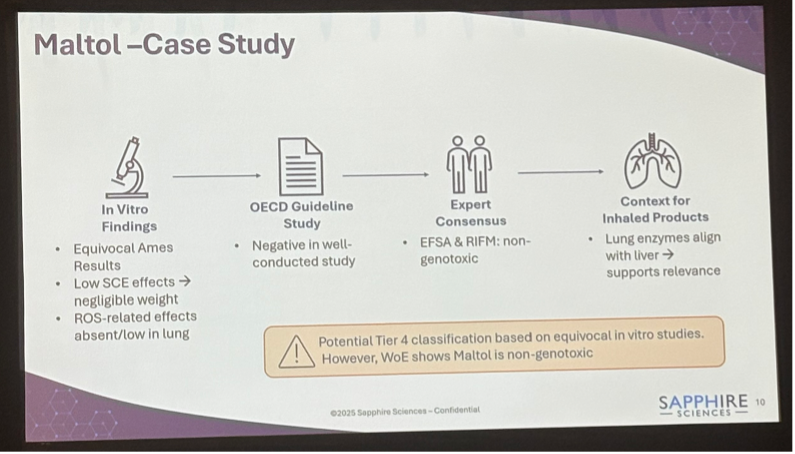
Evaluating FDA’s ELCR Framework: Strategic Insights for ENDS Product Assessments – Sapphire Sciences’ Ramez Labib unpacked FDA’s Excess Lifetime Cancer Risk (ELCR) framework with a case study on maltol, showing how a weight-of-evidence (WoE) review can re-tier equivocal Ames (mutagenic) results and avoid overinflating ELCR in ENDS assessments.
Cancer Potency for “Non-Mutagenic” Flavorings – Juul Labs’ Felix Ayala-Fierro presented potency estimates for β-myrcene and pulegone. He walked through the underlying calculations and highlighted how potency values can inform comparative assessments of ENDS ingredients.
Comparative QRA (Quantitative Risk Assessment) and Component-Based Risk Assessment for Evaluating the Relative Health Risks – Riskwise’s Autumn Bernal compared Breeze Pro disposable ENDS with both combustibles and FDA-authorized ENDS (NJOY DAILY Rich Tobacco and Menthol). The QRA of key constituents and the product as a whole, combined with in vitro findings indicated minimal toxicity of Breeze aerosols. These findings strengthened the WoE that both tobacco- and non-tobacco-flavored disposable ENDS may represent reduced-harm options.

Hazard Identification and Cumulative Risk Assessment of NJOY ENDS Products – Altria’s Sherwin Yan walked through hazard identification and cumulative risk assessment for NJOY ENDS. He highlighted the following limitations associated with CTP’s ELCR methodology:
- CTP’s ELCR approach may vastly overestimate actual human health risk. Their ELCR approach does not yield risk estimates aligned with other published analyses that have estimated 3-6.8% excess risk of ENDS to combustible cigarettes. CTP ELCR conservatively includes chemicals that may have sufficient (oral) in vivo data demonstrating a lack of carcinogenicity.
- CTP’s approach on hazard tiering depreciates the value of WoE approach by considering deficient studies (non-GLP, non-OECD) for an identified hazard endpoint in lieu of robust evidence.
- CTP has not made constituent/ingredient tiering publicly available – only obtained through FOIA.
Altria provided the following recommendations:
- Make the tox profiles and IURs (Inhalation Unit Risk) public, provide industry opportunities to review and comment, and/or provide additional data.
- Develop a system to engage stakeholders in a transparent process of compiling and reviewing data to establish proper IURs.
- Have clear criteria on the study qualities that can be included or excluded in the tox profile evidence.
Panel Discussion: From Product Development to Regulatory Decision-Making
The day closed with a risk assessment panel, “Quantitative Risk Assessment in Toxicological Risk Evaluation: From Product Development to Regulatory Decision-Making.” Moderated by Charlene Liu and Cassandra West, the discussion featured panelists Jonathan Fallica (PMI), Amy Madl (Valeo Science), Sherwin Yan (Altria), and Irene Abraham (JTI).

Amy Madl discussed the need for risk assessments that integrate both component-based QRA and mixture-based in vitro testing. Panelists emphasized the need for open dialogue with FDA, especially to clarify ingredient tiering for genotoxicity/carcinogenicity and weight-of-evidence criteria, and to address the memos’ concerns about mixture-based in vitro testing and cross-product genotoxic potency comparisons. Key takeaways included:
- Component-based QRA provides benchmarks against risk thresholds and comparator products.
- Mixture-based testing reflects real-world exposures and potential interaction effects.
- Genotoxic potency and dose-response can differ between single compounds and mixtures.
- Mechanistic understanding, dose-response data (e.g., NOGEL [No Observed Genotoxic Effect Level]), potency, and relevance to human exposure are critical for integrating genotoxicity into risk assessments.
Key Debates from the Panel
Several complex issues were discussed in depth:
1. Constituent Inclusion in ELCR Calculations. The ICH Guidelines (2023) link DNA-reactive substances that may cause mutations and cancer to positive Ames results. Non-mutagenic genotoxicants typically have threshold mechanisms and minimal carcinogenic risk at low levels. The FDA’s carcinogenicity tiering system extends beyond Ames mutagenicity (Tiers 4A and 4B). In addition, FDA previously indicated that QSAR-predicted (Tier 4D) and data-deficient constituents (4E) may not need to be included in ELCR calculations, aligning with ICH guidance. However, ambiguity in the FDA memo creates uncertainty.
Question: What is your opinion on including non-Ames positive in vitro (4C), QSAR-predicted (4D), or data-deficient (4E) constituents in the ELCR calculations if they lack confirmed mutagenic or carcinogenic potential? The overall consensus was not to automatically include 4C/4D/4E constituents in the ELCR by default, but instead to evaluate them with a WoE approach to avoid inflating ELCR.
2. Ingredient Data Gaps. Most e-liquid ingredients lack carcinogenicity evaluations and dose-response data from regulatory or professional agencies. In these cases, the Threshold of Toxicological Concern (TTC) of 1.5 µg/day is used as a surrogate to calculate ELCR.
Question: What is your experience in deriving a chemical-specific IUR or slope factor for data-poor constituents or using read-across? When inhalation data are missing, it may be beneficial to derive a chemical-specific IUR by transparently using read-across to closely related chemicals, identifying a Point of Departure (PoD), converting it to a human-equivalent concentration, applying appropriate uncertainty factors, and fully documenting the analog selection and rationale.
Question: What is your view on using the default TTC of 1.5 ug/day to quantify ELCR for such constituents, particularly QSAR-predicted (Tier 4D) and data-deficient (4E)? Defaulting to the TTC (1.5 µg/day) potentially inflates the final ELCR and dwarfs the contribution of known carcinogenic HPHCs.
3. Mixture-Based In Vitro Testing. FDA has historically criticized the use of mixture-based in vitro test articles compared to single chemical substances. Recent FDA memos express concerns about using standard in vitro assays for genotoxicity testing of ENDS. In the JUUL TPL (2025), FDA addressed positive in vitro findings for e-liquids and aerosols by applying ELCR calculations and appropriate hazard identification.
Question: What are the key considerations for designing an in vitro test to effectively address FDA’s concerns? Design in vitro studies to meet FDA’s expectations by using sufficiently high, scientifically justified dose ranges, fully characterizing the test sample, and following OECD test guidelines under GLP.
Question: Is in vitro testing still necessary for assessing the toxicological profile of ENDS and other tobacco harm reduction (THR) products? Yes, mixture-based in vitro testing of the whole ENDS aerosol (particulate + gas-vapor phases) remains necessary because it captures device- and matrix-driven interactions that single constituent assessments can miss. When interpreted alongside QRA of HPHCs and ingredient-specific data, it yields stronger, more decision-relevant weight-of-evidence for the toxicological profile and comparative risk of THR (Tobacco Harm Reduction) products.
Looking Ahead
TSRC 2025’s risk assessment sessions reinforced a shared need: aligning regulatory frameworks with robust scientific evidence while maintaining transparency. The combination of QRA, WoE approaches, and in vitro testing can deliver more realistic, decision-relevant assessments of ENDS and other tobacco harm reduction products.
For those designing studies or interpreting regulatory memos, the message was clear: dialogue with FDA is critical, criteria for evidence must be transparent, and ELCR calculations must not be inflated by default inclusion of data-deficient or low-quality evidence.
TSRC 2025 Wrap Up: Final Thoughts
The consensus was clear: risk assessments must balance regulatory caution with scientific rigor. With clearer criteria, greater transparency, and robust in vitro + QRA integration, the field can move toward more consistent, decision-relevant evaluations of ENDS and other THR products.
Contact Labstat for Toxicology Analyses
TSRC 2025 covered several important and interesting topics related to toxicology. If you have questions or want to discuss your toxicology needs, please contact us.




