The ToxTracker® assay is a valuable tool for evaluating the genotoxic profile of new products, providing critical insights into potential DNA damage, oxidative stress, and protein misfolding responses. Its ability to detect specific gene activation patterns under different conditions makes it highly effective in assessing the safety of novel products during development and a cost-effective screening tool for prototype screening.
This assay not only supports product innovation by identifying potential hazards early but also strengthens regulatory submissions by providing robust, mechanistic data that meet regulatory standards for safety evaluation.
Labstat and Toxys worked together to conduct a head-to-head study using the ToxTracker assay to evaluate the genotoxicity and cytotoxicity of different tobacco and nicotine products. We wanted to ensure that both labs showed similar results confirming the robustness of the assay and proficiency of our labs.
The results of the study demonstrated that both laboratories produced comparable findings across all tested products, indicating a high level of consistency and reliability in using the ToxTracker assay for assessing genotoxicity. The alignment in results across the labs provides confidence in the robustness of the assay and its applicability for evaluating a range of tobacco and nicotine products.
Overall, ToxTracker serves as a powerful asset in the safety assessment and regulatory approval process of new product development. Contact Labstat about testing using the ToxTracker assay.
Keep reading for more information.
Discussion of Results
Labstat and Toxys conducted a proficiency testing study using the ToxTracker® assay to evaluate the genotoxicity and cytotoxicity of different tobacco and nicotine products, including 1R6F combustible cigarette smoke (20 mg/mL in DMSO), CRP 2.1 moist smokeless tobacco reference (200 mg/mL in DMSO), and vanilla nicotine pouches (300 mg/mL in DMSO).
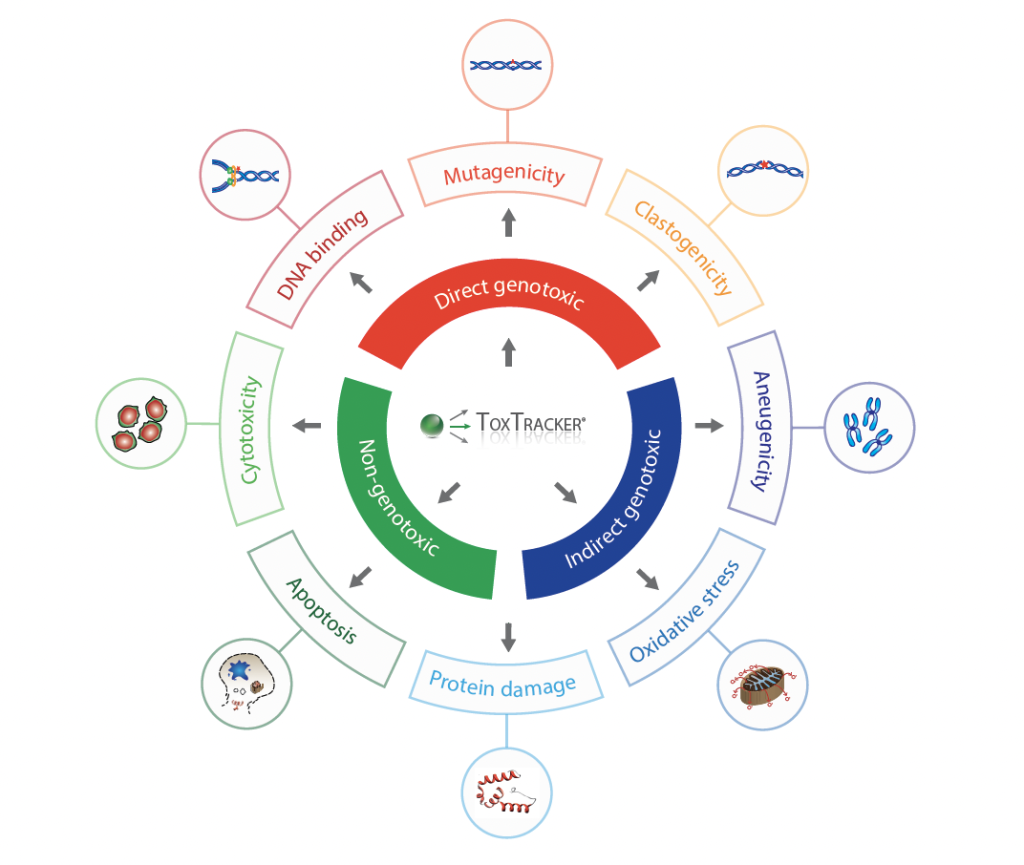
The ToxTracker assay is a high-throughput reporter-based genotoxicity test that detects DNA damage, oxidative stress, and protein misfolding by monitoring the activation of specific cellular pathways in response to chemical exposure. ToxTracker can identify both direct and indirect genotoxicity, as well as mechanisms of action related to non-genotoxic carcinogenicity.
Purpose of this Proficiency Study
The study aimed to compare the performance and consistency between the two labs in detecting and quantifying toxicological effects.
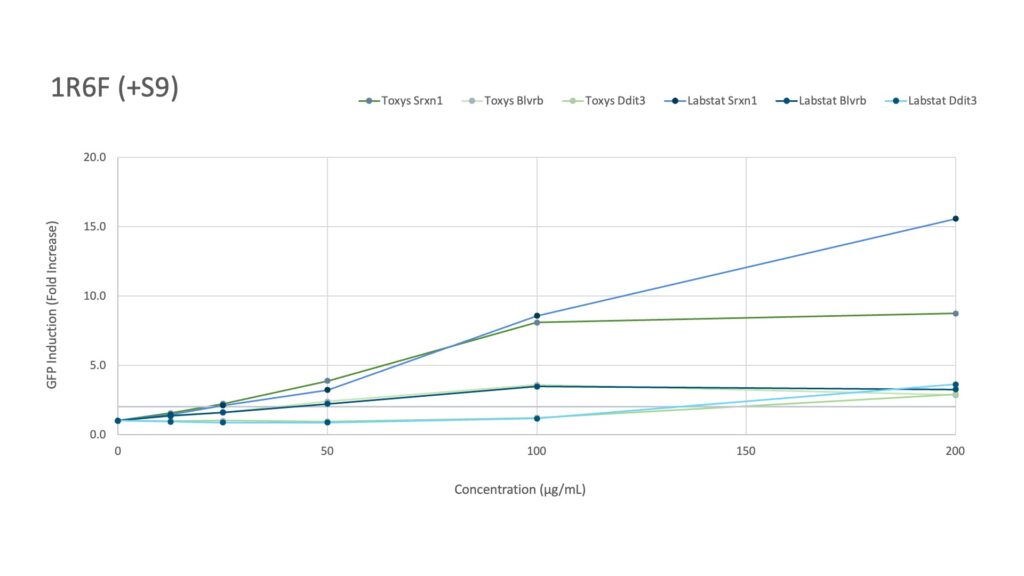
The proficiency study revealed distinct gene activation patterns for the tested samples. The 1R6F reference cigarette, both with and without metabolic activation, at the concentrations tested showed an increase in the expression of Srxn1, Blvrb, and Ddit3, indicating potential protein damage and oxidative stress responses.
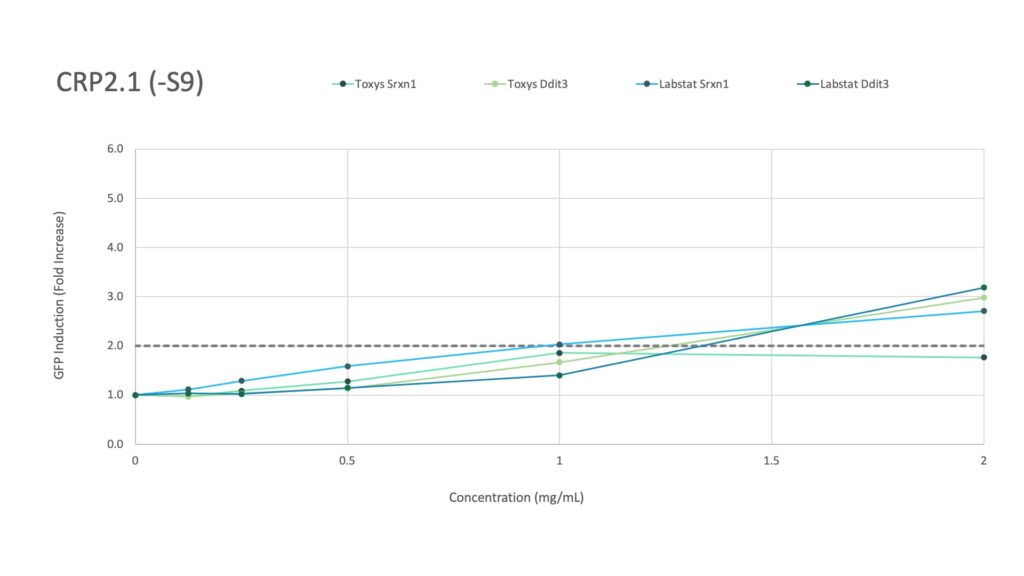
CRP2.1, a moist smokeless tobacco reference product, did not induce any gene response with metabolic activation, but without metabolic activation, an increase in Ddit3 and Srxn1 (Labstat only) was observed.
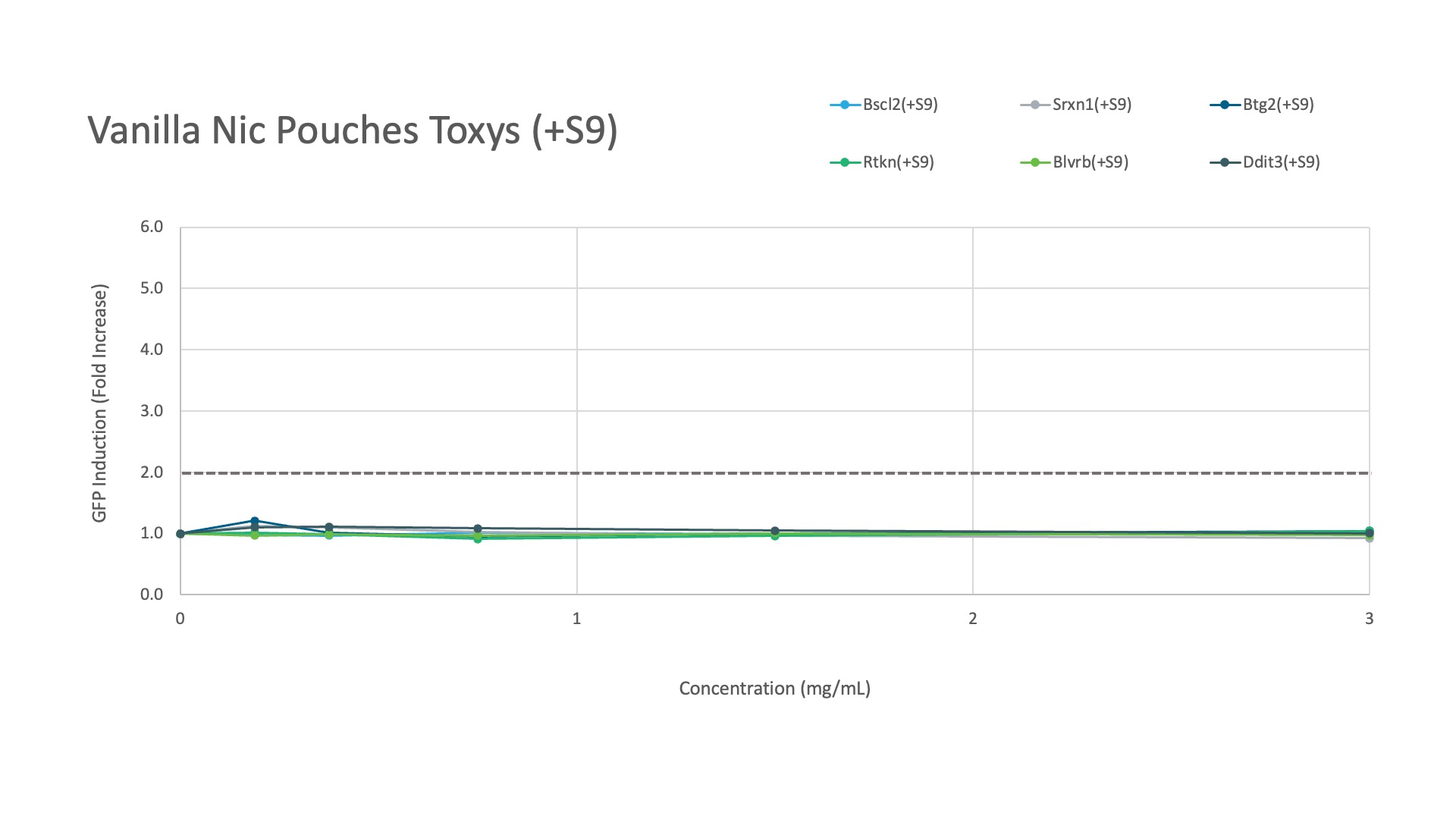
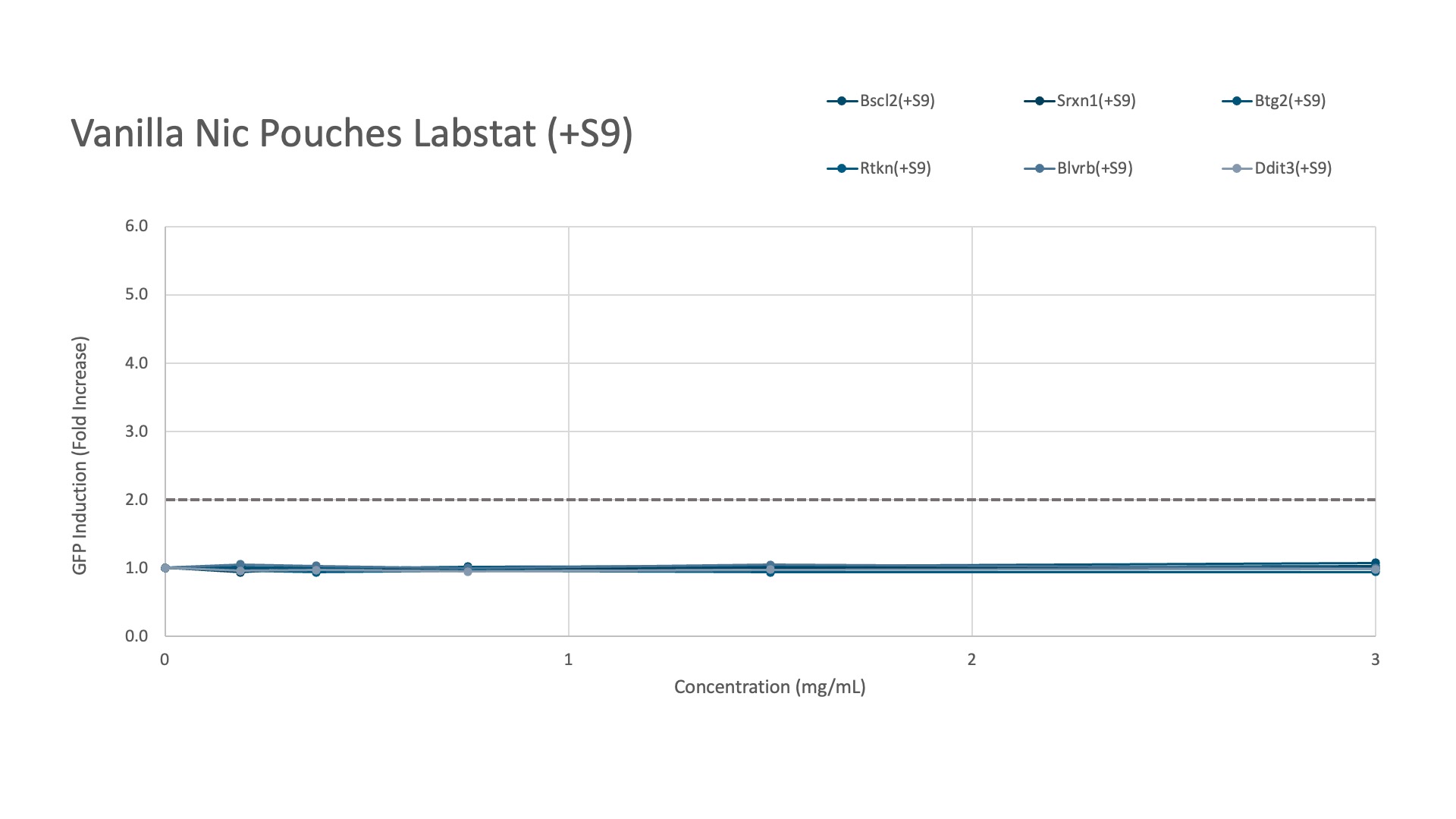
Meanwhile, the vanilla nicotine pouches showed no induction of any gene response, regardless of metabolic activation.
The following graphs show the results.