Identification of Ingredients in Supplements and NHP
There are two primary identification (ID) methodological approaches that are currently accepted by stakeholders involved in the Dietary Supplement industry:
- Chromatographic fingerprinting: High Pressure Liquid Chromatography (HPLC) and Gas Chromatography (GC) ID methods are highly respected techniques in the identification of Dietary Supplement bio-active ingredients because of the high degree of accuracy, precision, and significant global scientific consensus in its methodological applications. It is therefore a highly recommended technique by scientists and governments abroad. It is also a valuable way to both accurately quantify and ID the bio-active chemical constituent in various matrix types for one price unlike the more traditional TLC and IR methods.
- Infra-Red methodology: IR testing for more than 250 bio-active botanical/herbal ingredients as well as common nutritional parameters and active pharmaceutical ingredients (API’s) depending on the physical/chemical properties of the sample in question.
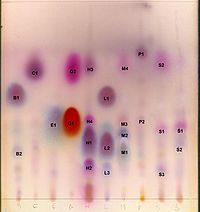
Thin Layer Chromatography Identification
TLC as a methodology is used to accurately identify (ID) high molecular weight, thermally labile, non-volatile solutes in mixtures.
Labstat offers TLC ID assays for more than 150 bio-active botanical/herbal ingredients as well as common nutritional parameters and active pharmaceutical ingredients (APIs).
Thin layer chromatography (TLC) is a traditional chromatographic technique that is used to identify bio-active ingredient mixtures by separating each bio-active ingredient and measuring its separation by its Rf Value.
TLC remains a highly respected identification technique in the Dietary Supplement Industry because of a long history of success with accuracy, precision, and significant global scientific consensus in its application. It is therefore a highly recommended technique by scientists and governments abroad.
Chromatographic Fingerprinting Identification
Chromatographic techniques such as Gas Chromatography (GC) and High Pressure Liquid Chromatographic (HPLC) technology are used to accurately and precisely quantify and identify (ID) organic compounds such as dietary supplement bio-active ingredient chemicals.
Labstat offers HPLC and GC assays for more than 250 bio-active botanical/herbal ingredients as well as common nutritional parameters and active pharmaceutical ingredients (API’s).
HPLC instrumentation is utilized for the quantification and ID those bio-active ingredients that are by nature polar, high molecular weight, thermally labile, non-volatile solutes in samples. Some common examples are the vitamins and bio-actives such as the ginsenosides in Ginseng roots.
GC instrumentation is utilized for the quantification and ID of non-polar (Lower polarity), lower molecular weight, volatile analytes in samples i.e. omega fatty acid EFA’s.
Infrared Identification
Infrared (IR) spectroscopy measures how well a sample absorbs light at each wavelength thereby exhibiting a specific characteristic infrared spectrum.
Labstat offers IR testing for bio-active botanical/herbal ingredients as well as common nutritional parameters and active pharmaceutical ingredients (API’s) depending on the physical/ chemical properties of the sample in question.
IR technology is categorized into 3 basic methodological techniques:
- Traditional Infra-Red- Dispersive and monochromatic
- Near Infra-Red (NIR)- Dispersive and monochromatic
- Fourier Transform Infrared spectroscopy (FTIR)- Polychromatic
The following table illustrates the wavelength ranges that decide which technique is best for the particular bio-active ingredient:
| Designation | Abbreviation | Wavelength |
|---|---|---|
| Near-Infrared | NIR | 0.78–3 µm |
| Mid-Infrared | MIR | 3–50 µm |
| Far-Infrared | FIR | 50–1000 µm |
IR instrumentation methods are limited to samples that constitute high purity or high homogeneity sample types.
Equipment and Supplies
Reliable laboratory equipment, appropriate reagents, representative samples, standard reference materials, and authenticated and in-house working plant reference materials are necessary to perform required assays.
Laboratory equipment and other apparatus (e.g., microscopes, sieves, slides, cover glasses, needles, and forceps) must be suitable to perform all required tests.
Laboratory equipment is calibrated at appropriate intervals and calibrations are documented.
Reagents and reference standards are of a quality and purity appropriate for the required tests.
Authenticated and in-house working plant reference materials are of a quality appropriate for the required tests.
Laboratory Quality
Laboratory certification is documented by appropriate accrediting organization if applicable, or by monitoring in-house quality assurance performance standards.
References:
U.S. FDA Center for Food Safety and Applied Nutrition: Ingredient Identity Testing Records and Retention, June 1999
W. C. Evans: Pharmacognosy, 14th Edition, 542-578
