 Pete Joza
Pete Joza  Pete Joza
Pete Joza1-Minute Summary
- Global demand for oral nicotine pouches is growing, and FDA actions (recent authorizations and a PMTA pilot) are shaping a clearer path to market.
- Nicotine dissolution testing shows how fast and how much nicotine a pouch releases – key for performance, R&D decisions, and regulatory submissions.
- Most studies use USP Apparatus 4 with validated artificial saliva; timelines and costs depend on SKUs, replicates, time points, and any method changes.
- Typical deliverables include release profiles and supporting notes you can use for PMTA packages, product optimization, and change control.
Oral Nicotine Pouch Growth Explodes
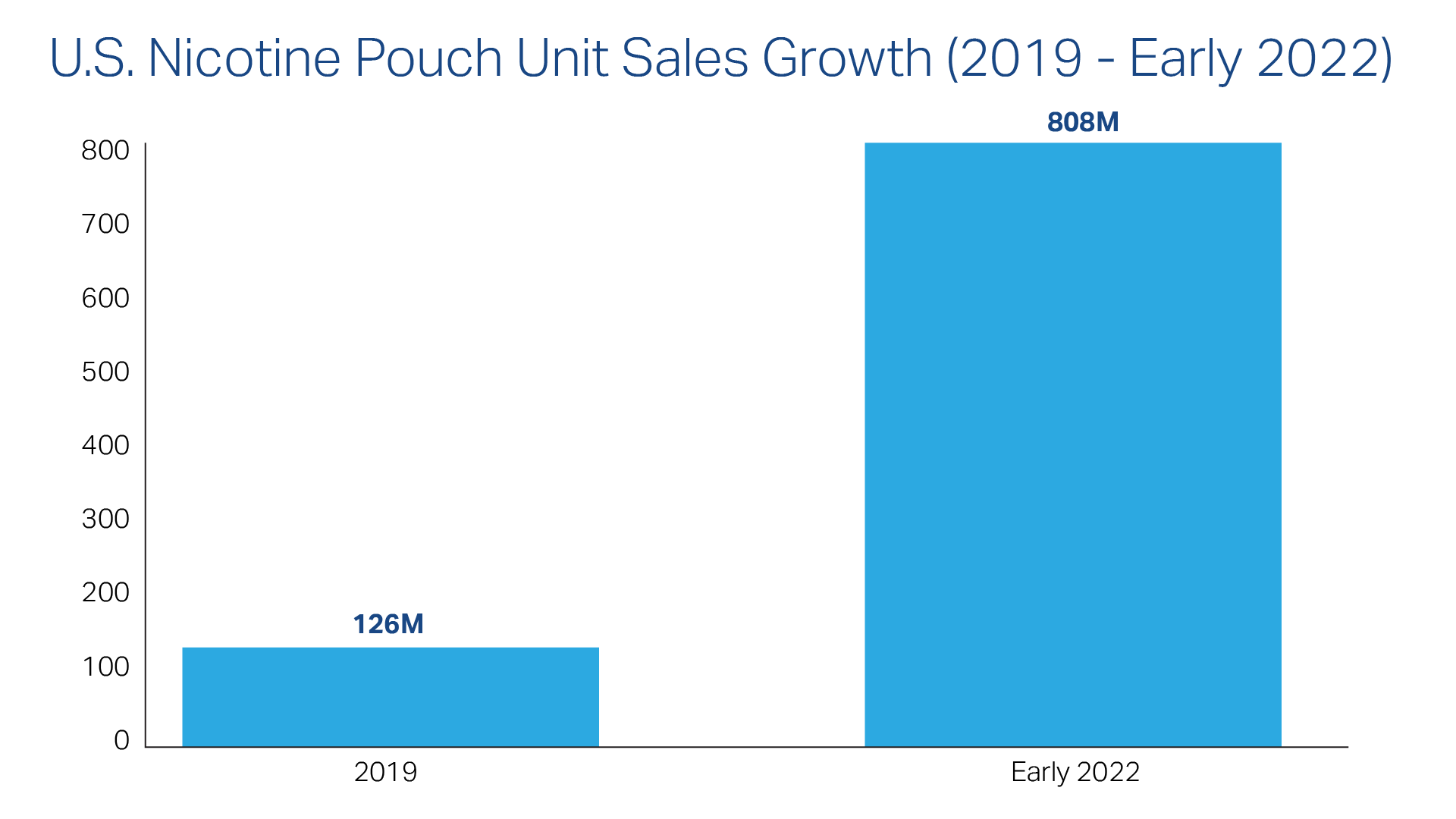
Everyone in the nicotine-product industry knows that sales of tobacco-free pouches have exploded since the late 2010s. The numbers bear this out. The JAMA Network reported that U.S. pouch sales grew from about 126 million units in 2019 to about 808 million by early 2022.
Industry insiders have good reason to expect the boom to continue. In January 2025, the FDA authorized marketing orders for 20 ZYN nicotine pouches. Then in September, the Agency announced a pilot program to streamline pouch PMTA reviews.
The promise of more products obtaining marketing orders means that analytical testing of pouch products will also grow in lockstep, particularly nicotine dissolution testing. In this article, we explain what you need to know about dissolution testing for your pouch products.
What is Nicotine Dissolution Testing?
Nicotine dissolution testing measures how quickly and how much nicotine is released from a pouch into a saliva-like medium over time at body temperature.
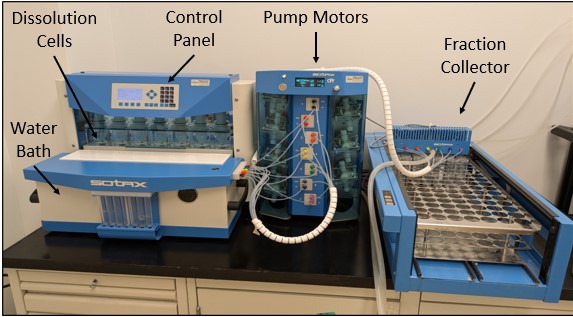
For modern oral nicotine products, labs typically use the USP Apparatus 4 (flow-through cell), collect time-based fractions for about an hour, and quantify nicotine by UPLC/LC-MS to generate a release curve.
Much research has been done in recent years into the nicotine-release profiles of pouches compared to other nicotine products, such as this Labstat study.
Our team has also researched the effect of salivary enzymes on nicotine release using nicotine dissolution testing.
How is Nicotine Dissolution Testing Performed?
A typical, fit-for-purpose study uses the USP Apparatus 4, as mentioned, with validated artificial saliva at ~37°C. Pouches are loaded into the flow-through cell; the medium flows at a controlled rate; fractions are collected at defined intervals (commonly up to ~60 minutes) and analyzed for nicotine to build a time-release profile.
This method is the industry standard for a few reasons:
- USP-4’s continuous flow helps maintain sink conditions and avoids floating/adhesion artifacts seen in static systems.
- The composition of artificial saliva (buffers, ions) influences pH and extraction; validated recipes (often DIN-based) and locked parameters support reproducibility.
- Parallel pH testing using CORESTA CRM 69 (scope extended to nicotine pouches) helps interpret the free-nicotine fraction, a key driver of perceived delivery.
Nicotine dissolution testing commonly uses n≈12 replicates per product and reports release curves alongside summary metrics.
How Long Does Nicotine Dissolution Testing Take & How Much Does It Cost?
Once everything is set, a single nicotine dissolution profile only takes about 60 minutes of fraction collection per unit.
However, much work can go into the set up, depending on project complexity. That’s why we often start with a scoping call to align on SKUs/strengths, replicate count (often 6–12), time points, and any method modifications your samples may need.
As you might expect, costs track with project scope. In general:
- Cost/Time Drivers: Number of SKUs, replicates, time points, potential method modifications, and add-ons (pH/free nicotine, moisture/water activity, etc.).
- Bringing your preferred SKUs, replicates, time points, etc., to the call will help speed the process. If you’re unsure, don’t worry – our team can help.
- Work with a dedicated nicotine-testing lab like Labstat, which has extensive experience performing nicotine dissolution testing using the USP Apparatus 4. This helps reduce rework or callbacks and supports your need for accurate, defensible data within your timeframe.
What Results Will I Receive?
Your results will depend on the project scope but in general you’ll receive a summary with visuals of how your pouches release nicotine over time. The goal is to show plainly how fast nicotine is released and how much within a typical use period. This data will help you make decisions about prototypes and products to meet regulatory and consumer demands.
What’s typically included (scope-dependent):
- A simple overview of findings and takeaways (e.g., whether products perform similarly or differently).
- Visuals or tables that show nicotine release over time for each SKU.
- Supporting notes on how the test was run, with enough detail for technical or regulatory review when needed.
How you’ll use it:
- Support PMTA and other regulatory submissions by documenting product performance.
- Guide product development (strength, flavor, pouch material).
- Manage changes (e.g., supplier or formulation updates) and show that performance stayed consistent.
If you’re unsure what level of detail you need, we can discuss during the scoping call.
Is Nicotine Dissolution Testing a U.S. FDA or Global Requirement?
United States (FDA)
For portioned smokeless products, including nicotine pouches, the PMTA rule requires reporting nicotine dissolution testing and pouch material information because these data are central to FDA’s scientific review.
The agency explicitly declined to remove these requirements in the final rule, underscoring their importance for understanding product performance. The codified PMTA content list also calls out nicotine dissolution rate and pouch parameters.
Outside the U.S.
There is no single harmonized global standard for pouches. Requirements vary widely by market, so manufacturers often run dissolution studies anyway to demonstrate product performance, comparability, and quality in technical dialogues with regulators and retail partners.
In many countries, providing the same information in a regulatory submission as required by a U.S. FDA PMTA satisfies their requirements. So, it’s a safe bet to include nicotine dissolution testing results in global regulatory submissions.
Ready to Plan Dissolution Testing?
All signs point to continued global growth in the nicotine pouch market, and dissolution testing is a major component of regulatory submissions and product R&D. If you have questions or are interested in scoping a project, contact our nicotine-testing experts today.


